Therapy of neuroendocrine neoplasias
Surgical therapy of neuroendocrine tumors:
Surgical procedure for neuroendocrine tumors of the small or large intestine
The treatment of neuroendocrine tumors can be divided into curative (="healing") and palliative ("symptom-relieving") interventions. Curative procedures include in particular the removal of small intestinal NET and appendix NET if there are no liver metastases. In most cases, parts of the small intestine are removed, sometimes together with the right-sided colon (right hemicolectomy).
Palliative interventions, e.g. the reduction of tumor mass in the case of pronounced carcinoid syndrome and in particular the removal of the primary tumor, often result in an increase in well-being for the patient, even in the case of extensive metastasis, e.g. in the liver. In addition, complications caused by the unhindered growth of the primary tumor are prevented. These include, in particular, tumor-related intestinal obstruction (ileus), intestinal bleeding or ingrowth of the tumor into vital organs or blood vessels.
The treatment of metastases, which are usually located in the liver, is also varied here and ranges from the surgical removal of large parts of the liver to local ablation using heat (radiofrequency ablation), cold (cryoablation) or by closing off the supplying vessels with chemotherapeutic agents via a catheter system (transarterial chemoembolization), which is usually inserted through the groin.
Surgical procedure for insulinoma
After biochemical confirmation of the insulinoma, surgery is always indicated. The only curative treatment method for an insulinoma is complete surgical removal. This permanently eliminates the symptoms. The intended procedure for benign insulinomas is enucleation (excision) of the tumor.
Surgical procedure for gastrinoma
Surgical treatment of gastrinomas is always indicated, even if imaging is negative, if there are no diffuse liver metastases. Standard therapy is the opening of the duodenum and resection of duodenal wall gastrinomas as well as enucleation of pancreatic gastrinomas in the head or left resection of tumors in the corpus-tail area. Apart from resection/enucleation of the primary tumor, extensive lymph node removal around the duodenum, pancreas and liver is necessary.
Surgical procedure for glucagonomas and VIPomas
VIPomas and glucagonomas should also undergo an aggressive surgical procedure.
Operative procedure for NFPTs
Surgical therapy plays the decisive role among the options for NFPT. The main goal is the complete removal of the tumor and its metastases, as this represents the only chance of cure for patients, relieves symptoms and increases the survival rate.
Small NFPT without evidence of metastasis should be removed in a parenchyma-sparing manner. In contrast, a partial pancreaticoduodenectomy (so-called Whipple's operation) should always be performed for larger NFPT in the area of the pancreatic head. The pylorus-preserving variant is now the method of choice. In the case of tumors of the tail of the pancreas, the tumor-bearing section of the pancreas is removed together with the spleen and lymph nodes.
Laparoscopic surgery for small NPTs is sensible, technically feasible and safe. However, it should only be planned and performed in centers with proven expertise in endocrine pancreatic surgery and laparoscopic surgery in general. Our clinic has been successfully performing laparoscopic surgery for NPTs for several years.
Interventional therapies: local ablative and loco-regional therapy procedures
Introduction
As a tumor disease progresses, tumor cells (metastases) spread to other tissues. Often, the first metastases are lymph nodes in the vicinity of the primary tumor. Then, in the case of malignant neoplasms of the gastrointestinal tract and the pancreas, the tumor cells spread via the draining veins and the portal vein to the liver. There, the tumor cells are filtered out due to the liver's special anatomy and liver metastases develop. Overall, the liver is the most common site of distant metastases in patients with neuroendocrine tumors (NET) of the gastro-entero-pancreatic system. Liver metastases can occur solitary (single), multiple (several, countable) or disseminated (diffuse, no longer countable). There are various approaches to treating liver metastases. In addition to conventional therapies such as surgery or chemotherapy, there are liver-specific procedures to reduce the tumor burden in NETs. These include various loco-regional procedures, which are explained below.
Local ablative procedures
Image-guided percutaneous radiofrequency ablation (RFA) is the most widely used and best evaluated local ablative method. In this method, tumor tissue is heated with a probe through an access through the skin (percutaneously) and thus destroyed. However, not all liver metastases are suitable for RFA. The number and size of the metastases, as well as the tumor biology and the experience of the therapist, play an important role here. RFA, together with surgery, can represent a potentially curative (aiming at healing) approach or can help to destroy as much tumor tissue in the liver as possible. RFA can be used repeatedly and has a low complication rate. Alternatives to RFA include microwave ablation or percutaneous cryotherapy.
Loco-regional procedures - transarterial embolization and chemoembolization
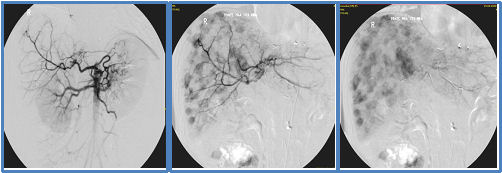
Liver metastases from NET (see Fig. 1) are very well supplied with blood and are supplied >90% by the hepatic artery (see Fig. 2). The surrounding healthy liver tissue, on the other hand, only receives about 20% of its blood supply from the hepatic artery and about 80% from the portal vein (V. portae). These tumor-specific properties are used in transarterial forms of therapy such as selective hepatic transarterial embolization (TAE) or transarterial chemoembolization (TACE).
For this purpose, the femoral artery is punctured under local anesthesia and a catheter is inserted into the hepatic artery via it. First, the blood supply to the liver metastases is visualized using contrast medium under X-ray fluoroscopy (see Fig. 2). The artery that supplies the tumor in the liver can then be closed (embolized) using various particles. Selective embolization (TAE) leads to a targeted reduction in blood flow (ischemia) of the tumor tissue.
TACE (transarterial chemoembolization) is based on the same considerations as TAE and is similar to it. However, with TACE, a chemotherapeutic agent is also selectively administered into the artery that supplies the tumor. This allows a very high concentration of the chemotherapeutic agent to be achieved in the tumor tissue. In addition, the effect of the chemotherapy is increased by the subsequent embolization of the vessel. Modern particles are now available that release the chemotherapeutic agent specifically in the tumor ("drug eluting beads"; DEB).
Both procedures (TAE and TACE) are used to reduce the tumor load in the liver or to alleviate the symptoms caused by the hormone release in functional NETs.
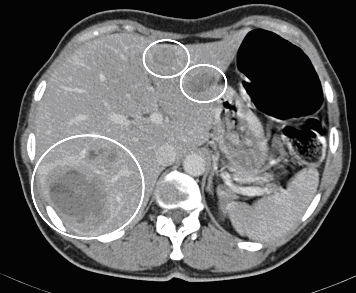
Computed tomography (CT) with contrast agent (CM) showing several liver lesions suspected of metastases (white rings). This CT was also used to plan a TACE depending on the location of the metastases.
Loco-regional procedures - radioembolization
Selective intra-arterial radiotherapy (radioembolization) uses transarterial access to the tumor vessels to carry out targeted radiation therapy. For this purpose, resin or glass particles to which a radioactive element (e.g. yttrium) is coupled are applied specifically to the liver. As with TAE/TACE, the principle of local intra-arterial radiation is based on the fact that the liver metastases are mainly supplied via the hepatic artery, while healthy tissue is mainly supplied via the portal vein. Here, too, the hepatic artery is accessed via an access in the groin. In addition to TAE/TACE, bypass circuits are prevented during this treatment. After an initial test run to check the possible distribution of the radioactive particles in the liver, the liver metastases are treated in a second session. While the healthy liver tissue is largely spared, radioembolization irradiates the tumor with very high doses that cannot be achieved with conventional radiotherapy.
Endoscopy
We routinely carry out interventional procedures on the bile duct system and pancreas, such as stent implantations and cyst drainage, with great expertise.
Due to many years of caring for NET patients, we also have extensive experience in the local removal of localized small NETs in the gastrointestinal tract. Modern, high-performance endosonography devices are available for endoscopic ultrasound diagnostics including punctures.
Biotherapy
So-called biotherapy with somatostatin analogues is often the first therapy used in patients with metastatic neuroendocrine tumors. It is based on the presence of somatostatin receptors (docking stations for somatostatin) on the tumor cell. The effect of these drugs is twofold. In functionally active tumors that produce hormones such as serotonin, the secretion of hormones is reduced. This can significantly improve symptoms that are based on hormone secretion, such as flushing or diarrhea. In addition, two studies (PROMID study and CLARINET study, the first was conducted under the leadership of the Marburg NET team on patients with NETs of the small intestine, the second on a broader patient collective including patients with NETs of the pancreas) have shown that these drugs also have an antiproliferative effect, i.e. they can actively slow tumor growth. There are currently two approved drugs in this substance group: Octreotide-LAR and Lanreotide-Autogel. Octreotide and Lanreotide are usually very well tolerated. Mild side effects such as diarrhea, flatulence or the formation of gallstones occasionally occur. Treatment is usually carried out with monthly depot preparations intramuscularly (Octrotide-LAR) or subcutaneously (Lanreotide-Autogel). In particular in the case of slowly growing NENs of the small intestine, the administration of somatostatin analogues is often successful in the long term as monotherapy. α-Interferon has been used successfully in the treatment of small intestinal NETs and has both antisecretory and antiproliferative effects, but has lost importance in recent years due to the frequent side effects compared to other treatment options, but can certainly represent a treatment option for small intestinal NENs in individual cases.
Chemotherapy
Chemotherapy is used successfully in the following two subgroups of NEN: Firstly, neuroendocrine carcinomas (NEC), which are a very fast-growing subtype of NEN that behave biologically more like small cell bronchial tumors. Here, a treatment consisting of a platinum-containing agent (cisplatin or carboplatin) with etoposide is classically used. In most cases, NEC responds well initially, but often relapses quickly after treatment is terminated or interrupted. Schemes containing irinotecan, for example, are used as second-line therapy. In the case of well-differentiated NENs, chemotherapy only plays a role in tumors that originate in the foregut, such as the pancreas, bronchus and stomach. For a long time, intravenous chemotherapy with streptozotocin (side effects: nephrotoxic (kidney damage), nausea, vomiting, slight changes in blood count) in combination with 5-FU has been used for these tumors, and is usually well tolerated. An alternative therapy is dacarbazine (side effects: slight changes in blood count, nausea, increased liver values), which is infused once a month and has a favorable side effect profile. A substance related to darcabazin that is given as a tablet is temozolomide. This is usually combined with a second chemotherapy tablet, capecitabine. For patients with pancreatic NET, good results can be achieved with intravenous chemotherapy with streptozotocin and 5-FU, which is formally approved for this purpose, as well as with tablet combination chemotherapy, which is not formally approved (partial remission, i.e. relevant shrinkage according to radiological criteria in 35-40%, plus 35-40% stable disease). The combination of temozolomide and capecitabine is also used successfully for so-called NET G3 - i.e. well-differentiated tumors with an increased proliferation rate Ki67 > 20%. With all chemotherapies, we ensure optimal concomitant therapy so that side effects are as low as possible. Chemotherapy plays no role in NET of the small intestine, as these are largely chemoresistant and the response rates are low.
Molecular Targeted Therapy
Two substances, everolimus and sunitinib, have been approved for several years. They specifically block certain intracellular mechanisms in the tumor cell. Both substances are available as tablets. Everolimus is a so-called mTor inhibitor, and sunitinib is a tyrosine kinase inhibitor. Both substances have been tested in randomized, double-blind studies, so there is clear evidence of effectiveness. In progressive (growing) NEN, these substances lead to a doubling of progression-free survival (the time during which the tumor does not grow). Sunitinib is only approved for the treatment of NET of the pancreas. Everolimus is also approved for NET of the gastrointestinal tract and lungs. Typical side effects of everolimus include changes in the blood count, worsening of pre-existing high blood pressure or diabetes, diarrhea, aphthous ulcers (open sores in the mouth) and pneumonitis (changes in the lung tissue). In addition to changes in blood count, sunitinib can cause loss of appetite, gastrointestinal side effects, increased blood pressure and pronounced tiredness (fatigue) and, rarely, hand-foot syndrome, taste disorders and hair discoloration. Monitoring and, if necessary, treating side effects is therefore important. Numerous other drugs from the group of tyrosine kinase inhibitors have been tested. An American phase III study demonstrated the effectiveness of cabozantinib compared to placebo in previously treated patients who suffered from progressive NET of the pancreas (pancreatic cohort) or the gastrointestinal tract and lungs (extrapancreatic cohort). Approval of the drug has been applied for, but has not yet been granted (as of 1/25). Here too, high blood pressure is one of the most common side effects, along with changes in blood count, diarrhea and fatigue.
Nuclear Medicine Therapy of Neuroendocrine Neoplasms
Peptide receptor radiotherapy (PRRT)
As in the field of diagnostics, the primary goal in therapy is to transport as much radioactive substance (also called radiopeptide or radioligand) as possible into the cells and to allow it to work from there.
If the radioactive substance is a substance that predominantly emits gamma rays, such as 111Indium, it is well suited for diagnostics. If, on the other hand, it is a predominantly beta emitter, such as 90Yttrium or 177Lutetium, these are suitable for therapeutic purposes.
The basis of the most commonly used radioligands is the substance DOTATOC or DOTATATE. There are several radioactive emitters to choose from, which are incorporated into the so-called chelator DOTA. Therapy with 90Yttrium or 177Lutetium is common today.
Requirements
The prerequisite for carrying out the therapy is that several locations of an organ (for example the liver) or several organs are affected, because surgery is the method of choice for individual lesions. Ultimately, only surgery can completely and permanently cure NEN diseases. Some other laboratory requirements must also be clarified in order to avoid side effects on the kidneys or blood count. The kidneys are always additionally protected by an amino acid solution during therapy.
The basic requirement before any therapy with DOTATOC/DOTATATE is that the tumor strongly accumulates the substance DOTATOC/DOTATATE, which is examined beforehand by scintigraphy.
Effectiveness of radioligand therapy
There are various European studies on the effectiveness of the therapy. In summary, it can be said that significant regression of more than 50% of the volume can be expected in about 20% of therapies, smaller regressions (tumor regression < 49% and > 25%) are detectable in about 10-20% of cases, while stable tumor behavior (SD) is the most common result in about 50% and is considered a therapy success.